innovativehealthsolutions
Latest
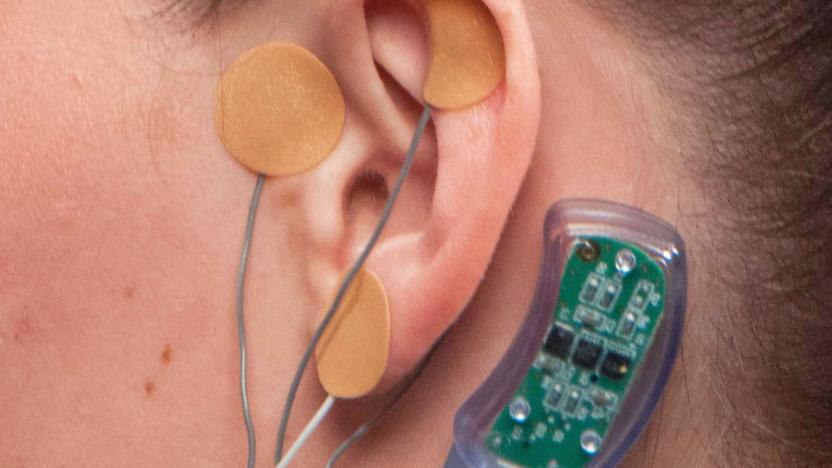
FDA approves an electrical device to help ease opioid withdrawal
Amid an epidemic of opioid addiction, doctors are looking for ways to help people wean themselves off of the drugs, but withdrawal symptoms are a major hurdle. Innovative Health Solutions says its NSS-2 Bridge is a "percutaneous nerve field stimulator (PNFS) device system" that sends electrical pulses to certain cranial nerves, treating symptoms including sweating, tremors, stomach upset, joint pain and anxiety. Yesterday the FDA cleared it for marketing, making this the first device approved for use in this way. Like the DEKA Arm System, this device was reviewed through the agency's de novo pathway that fast-tracks "some low- to moderate-risk devices." Prior to approval, the FDA reviewed a clinical study of 73 patients where all of them showed at least a 31 percent drop in their clinical opiate withdrawal scale (COWS) score within 30 minutes of use.
