sherlock biosciences
Latest
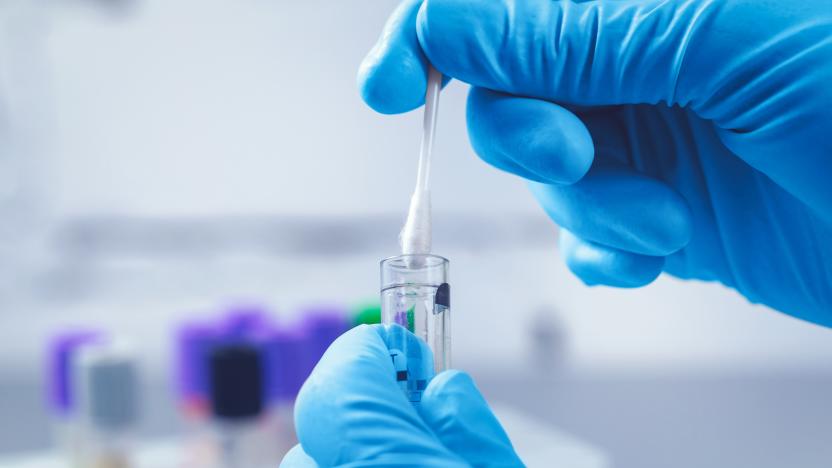
FDA approves a rapid COVID-19 test that uses CRISPR
Sherlock Biosciences has received an Emergency Use Authorization (EUA) from the FDA for a rapid COVID-19 test that uses CRISPR technology. Sherlock’s CRISPR SARS-CoV-2 test uses a CRISPR molecule to detect the genetic signature of the virus. The kit, which uses a nasal swab or bronchoalveolar lavage (BAL) specimen, is designed for use in laboratories authorized to perform high complexity tests.
