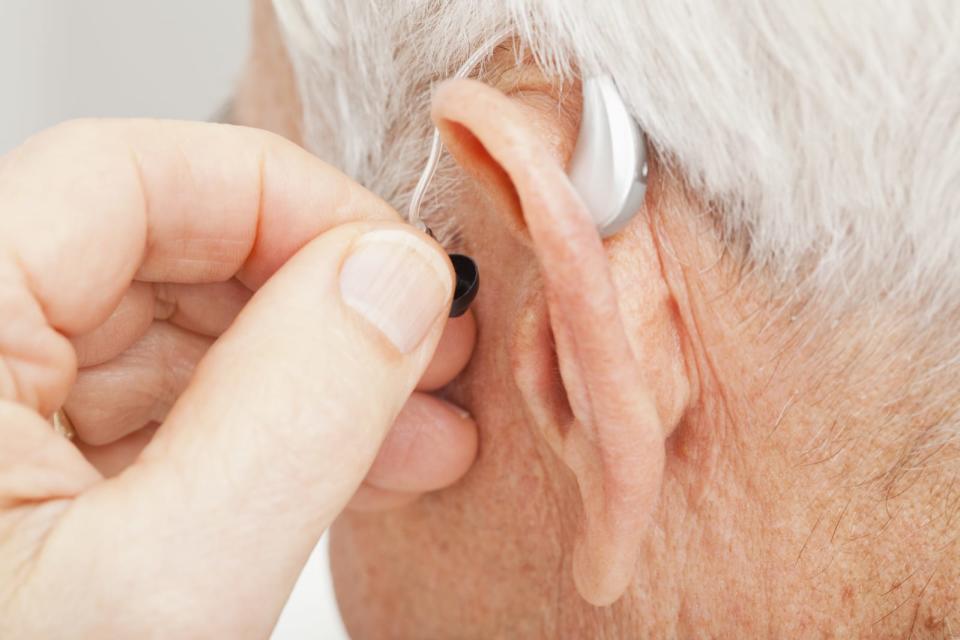
FDA approves direct-to-consumer hearing aid from Bose
It’s the first hearing aid that can be fit and programmed by the user.
The US Food and Drug Administration has, for the first time, approved a hearing aid that can be fit, programmed and controlled by the user instead of a healthcare provider. The device comes from Bose and users can make adjustments to its settings in real time through a mobile app.
"Hearing loss is a significant public health issue, especially as individuals age," said Malvina Eydelman, director of the Division of Ophthalmic, and Ear, Nose and Throat Devices at the FDA's Center for Devices and Radiological Health. "Today's marketing authorization provides certain patients with access to a new hearing aid that provides them with direct control over the fit and functionality of the device. The FDA is committed to ensuring that individuals with hearing loss have options for taking an active role in their health care."
The US passed a new law last year that will allow for over-the-counter hearing aids, and it aims to provide adults with mild to moderate hearing loss access to hearing aids without them having to go through a physician first. The FDA is currently working on regulations for the new category of hearing aids. The agency says around 37.5 million adults report hearing loss ranging from "a little trouble" to "deaf."
Though they're not approved by the FDA as hearing aids, a number of companies have developed wireless earbuds that can manipulate and augment sound. Bose, Nuheara and the now defunct Doppler Labs have all released assistive hearing devices in the past.
Update 10/11/18 5PM ET: This post was updated to reflect that the Bose Hearing Aid is not considered an over-the-counter device by the FDA, but is instead a direct-to-consumer device. The FDA is still working on drafting regulations regarding over-the-counter hearing aids, a new category allowed by a law passed last year.