fda
Latest

Study backs blood test that gauges seriousness of concussions
Remember how the FDA approved a blood test that could determine the severity of a concussion? It's now clear just why the FDA gave its approval. The Lancet Neurology has published the study at the heart of the decision, giving you a chance to verify the claims for yourself (if you're willing to pay, at least). As before, Banyan Biomarkers' test checks for the presence of two brain proteins (GFAP and UCH-L1) whose levels rise when there are signs of internal trauma. The FDA's claims check out -- out of 1,959 eligible test subjects, just three had CT scans turn up results when the blood tests were negative.

FDA declares meat-free Impossible Burgers safe to eat
The Food and Drug Administration has finally given Impossible Burger's plant-based meat its stamp of approval. Impossible Foods submitted the meat substitute for review back in 2014, but the FDA responded with concerns that its key ingredient, a protein known as soy leghemoglobin, might cause allergies and other adverse effects. The protein is commonly found in soy plants' roots, but since we don't typically eat that part of the plant, the FDA had reservations about its safety. In response, the company sent in more info, including results from a rat-feeding study, which convinced the agency to declare that the plant-based meat (and soy leghemoglobin) is "generally recognized as safe" for human consumption.

FDA approves its first marijuana-derived drug
In a nationwide first, the US Food and Drug Administration (FDA) has authorized the use of 'Epidiolex', a marijuana derivative which will be used to treat rare forms of epilepsy. Epidiolex -- also known as cannabidiol or CBD -- is a highly-purified version of the many psychoactive compounds found in marijuana, and does not result in a high.

FDA approves implantable glucose monitor that lasts for 90 days
A year ago, the FDA implemented new, streamlined regulations for digital devices. One of them is a continuous glucose-monitoring (CGM) system the FDA just approved that sends data from an implantable sensor to a paired mobile app, letting patients see their levels on their smart device. Once it's inserted by a medical professional, it can stay in the body for up to 90 days, which is far longer than comparable external sensors that typically must be swapped out around the ten-day mark. It's the first implantable CGM approved by the agency.

FDA approves AI tool for spotting wrist fractures
The FDA has been approving its fair share of AI-powered medical technology, but its latest might be particularly helpful if you ever have a nasty fall. The agency has greenlit Imagen's OsteoDetect, an AI-based diagnostic tool that can quickly detect distal radius wrist fractures. Its machine learning algorithm studies 2D X-rays for the telltale signs of fractures and marks them for closer study. It's not a replacement for doctors or clinicians, the FDA stressed -- rather, it's to improve their detection and get the right treatment that much sooner.

FDA approves AI-powered software to detect diabetic retinopathy
30.3 million Americans have diabetes according to a 2015 CDC study. An additional 84.1 million have prediabetes, which often leads to the full disease within five years. It's important to detect diabetes early to avoid health complications like heart disease, stroke, amputation of extremities and vision loss. Technology increasingly plays an important role in early detection, too. In that vein, the US Food and Drug Administration (FDA) has just approved an AI-powered device that can be used by non-specialists to detect diabetic retinopathy in adults with diabetes.

FDA wants Facebook and Twitter to crack down on opioid sales
In a speech given yesterday at the National Rx Drug Abuse and Heroin Summit, FDA Commissioner Scott Gottlieb announced that the agency would be inviting a number of internet company CEOs to a summit that will host discussions on potential solutions to the tech industry's role in the US opioid crisis. Gottlieb says that the FDA has found offers to purchase opioids and other drugs on Twitter, Facebook, Instagram, Reddit, Google, Yahoo and Bing. He added that in a report from the US Senate Permanent Subcommittee on Investigations, investigators found that "'it was easy to find fentanyl advertised online,' pay for it using cryptocurrency or credit cards and have it shipped to anywhere in the United States through international mail."

Medicare now covers gene sequencing for patients with advanced cancer
Patients with advanced cancer will soon have access to more personalized treatment plans because Medicare will now cover genetic tests that sequence tumor cell DNA and help healthcare providers determine treatment strategies. As Wired reports, the Centers for Medicare & Medicaid Services (CMS) announced that for Medicare-enrolled patients with recurrent, metastatic, relapsed, refractory or stages III or IV cancer, such FDA-approved tests will be covered by their insurance. And since other insurance companies tend to take their cues from CMS, privately-insured patients will likely get similar coverage soon as well.

Amazon hires former FDA exec for secret health care team
Amazon has made a big hire for its secret health care division internally known as 1492, according to CNBC: FDA's first chief health informatics officer Taha Kass-Hout. The e-commerce giant reportedly brought him on board to work under former Google X director Babak Parviz in a business development role. What that role is remains a mystery -- it is a secret division, after all -- but based on Kass-Hout's previous jobs, CNBC believes he might help Amazon conjure up a way for people to get easier access to their health records.

FDA greenlights 23andMe's direct-to-consumer cancer risk test
Cancer screening isn't all that accessible -- you typically need an obvious genetic background that suggests you're at risk, which doesn't help if you slip between those cracks. You shouldn't have to run that gauntlet for much longer. The US Food and Drug Administration has approved a 23andMe direct-to-consumer test that details the risks of breast, ovarian and prostate cancer based on BRCA1 and BRCA2 genetic mutations. Once the report is available, you wouldn't have to worry about qualifying for a screening -- you could send in a saliva sample and find out on your own terms.

FDA approves blood test that determines severity of concussions
The FDA announced this week that it has approved a blood test that can quickly and reliably detect signs of a concussion. Typically, when someone seeks medical care following a head injury, they're subjected to a neurological test and/or a CT scan. However, CT scans can only detect bleeding or swelling in the brain, and for injuries that are more minor but still serious, those scans aren't terribly useful. Additionally, if CT scans don't spot anything, and in many cases they don't, the person undergoing the scan has been subjected to unnecessary radiation and, usually, an unneeded expense.

Studies suggest cellphone radiation doesn't threaten humans
No, the debate over the risks of cellphone radiation isn't over yet. The US National Institutes of Health's National Toxicology Program has published details of draft studies which suggest that normal cellphone radiation levels aren't harmful to humans. The research subjected rats to very high levels of RF radiation at 2G and 3G cellular frequencies, and produced results where there was no clear pattern of harm even at the exaggerated radiation levels.
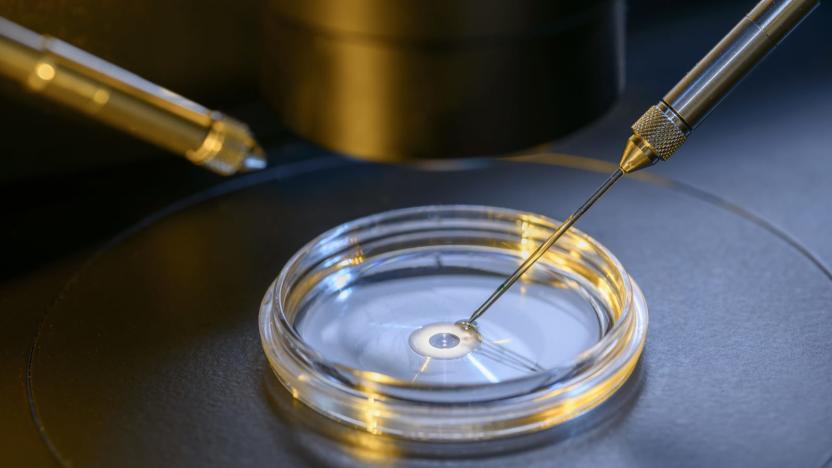
UK doctors plan country's first three-person fertilization procedure
UK officials have approved two women for mitochondrial replacement therapy, the fertilization procedure that results in a baby technically parented by three people, the Guardian reports. The procedure, which still isn't approved in the US, was legalized in the UK in 2015 and the Newcastle Fertility Centre, where the two women will be treated, was granted a license to perform the procedure last March. While it's not the first time a procedure like this has been done, it is the first time it will happen in the UK.

Gene therapy treatment for hereditary eye disease will cost $850,000
Last month, the FDA approved a gene therapy called Luxturna, which can treat a rare eye disease that causes blindness. Now the treatment has a price tag, CNBC reports. It will cost $425,000 per eye, and while $850,000 is steep, it's lower than the $1 million at which many expected the treatment to be priced.

FDA approves first shock wave device made to heal wounds
Using "acoustic shock waves" to promote healing isn't just for Overwatch, as Sanuwave has obtained FDA approval for its Dermapace System (Pulsed Acoustic Cellular Expression = PACE). Its approval is specifically to help heal foot ulcers in diabetic patients, where damage to blood vessels and nerves can lead to reduced circulation, infection and sometimes amputation. The Dermapace mechanically stimulates the wound, which Sanuwave says promotes healing. Like several other "first" FDA approvals we've seen recently, this device went through the de novo review process designed specifically to get new technology on the market.

FDA OKs first gene therapy for hereditary disease
The FDA has approved a new gene therapy that proves the technique can also be used to treat a variety of diseases other than cancer. According to the agency's announcement, the therapy called Luxturna can treat biallelic RPE65 mutation-associated retinal dystrophy, a rare eye disease that leads to blindness, in both adults and children. This is the first time the agency approved a gene therapy designed to treat a hereditary illness and the first to target a disease caused by mutations in a specific gene -- it's a major milestone in the field and a huge victory for its proponents.

FDA clears first EKG band for the Apple Watch
AliveCor's KardiaBand, a device that detect dangerous heart rhythms, has become the first Apple Watch accessory cleared for medical use by the FDA, the company announced. It can capture your EKG in 30 seconds, then detect problems like atrial fibrillation, a type of heart arrhythmia.
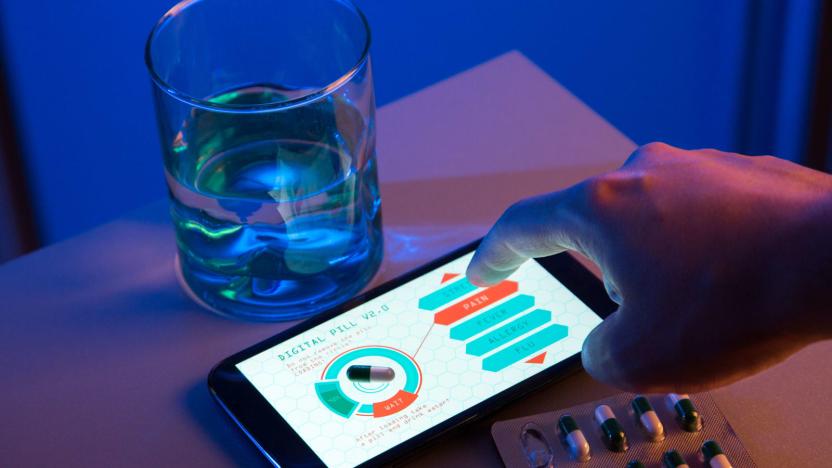
FDA approves first drug that doctors can track remotely
Doctors in the US might soon start prescribing a pill that can tell them whether you've truly taken your medication. The Food and Drug administration has approved the country's first digitally tracked medicine to ensure patients comply with their prescriptions, since non-compliance is a costly problem that could negatively affect a patient's health. Still, medical professionals are raising concerns about using pills that can be tracked, especially because, for some reason, the drug the FDA approved is an antipsychotic used to treat schizophrenia and bipolar disorder.
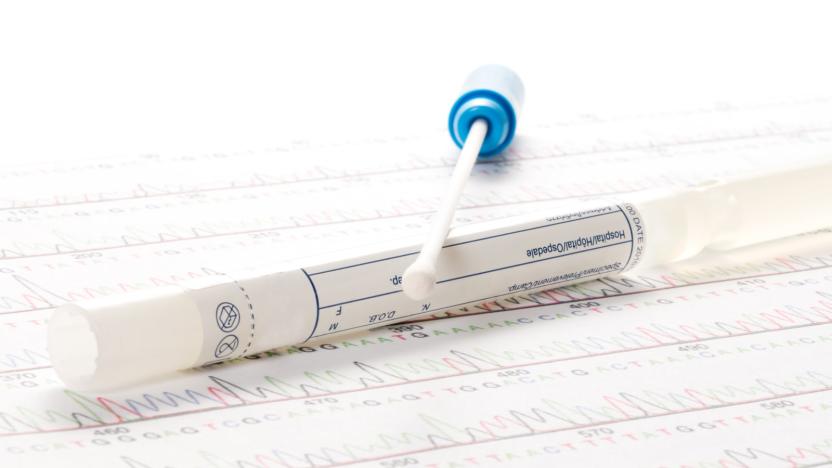
The FDA just changed how it reviews genetic health risk tests
FDA Commissioner Scott Gottlieb announced new rules today regarding direct-to-consumer genetic health risk (GHR) tests and the process by which they're approved for sale. In a statement, Gottlieb explained that these sorts of tests can provide more and more information as the technology develops, information that is not only in demand but could also serve as a useful medical tool. "These tests can prompt consumers to be more engaged in pursuing the benefits of healthy lifestyle choices and more aware of their health risks," said Gottlieb. "Consumers are increasingly embracing genetic health risk testing to better understand their individual risk for developing diseases."

Five-minute allergy test passes the FDA's scrutiny
A few years ago, researchers from the Swiss Federal Institute of Technology in Lausanne (EPFL) started developing what they eventually dubbed the "world's most rapid" allergy test. Now, that test has received the FDA's approval and will start telling you what you're allergic to in as fast as five minutes next year. Abionic, the EPFL spinoff that took over the test's development in 2010, created the abioSCOPE platform and its accompanying single-use test capsules to be able to detect your allergies with just a single drop of blood.







