fda
Latest

Gene therapy for advanced lymphoma gets FDA approval
People with advanced lymphoma now have another type of treatment to consider. The Food and Drug Administration has approved Yescarta, a cell-based gene therapy designed to treat large B-cell lymphoma originally developed by the National Cancer Institute*. It's the second time the FDA has approved a gene therapy for use in the US following a procedure meant to treat leukemia earlier this year.
FDA clears implant that treats severe sleep apnea
Sleep apnea (where your brain doesn't properly send breathing signals while resting) is horrible enough by itself, but the solutions to it can be scary: you may have to take medication, rely on ungainly breathing machines or opt for invasive surgery. You might have a gentler treatment going forward, though. The US Food and Drug Administration has approved an implantable device, Respicardia's Remede System, that fights more serious cases of sleep apnea.

FDA approves first Zika test for blood donations
While the Zika virus is primarily transmitted by mosquito bites, it could also be passed through blood transfusion. To ensure that nobody who needs transfusion in the US gets infected, the US Food and Drug Administration has approved the first test that can screen Zika in blood donations. The cobas Zika test can detect the virus' RNA in plasma taken from donated whole blood and blood components. It can't be used to diagnose infection, but it can keep the country's blood supply Zika-free.
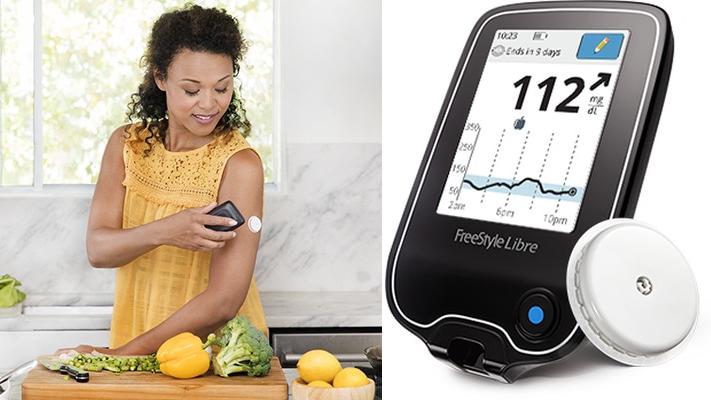
FDA OKs a blood sugar monitor that doesn't need fingerpricks
A fingerprick isn't just a fingerprick when you have to do it all the time to test your blood sugar levels. Thankfully, the Food and Drug Administration has approved the first continuous glucose monitoring system for adults that doesn't require you to draw blood several times a day. Abbott's FreeStyle Libre Flash Glucose Monitoring System works by inserting a tiny sensor wire below the surface of your skin. The wire needs 12 hours to start up, but once it's ready, you can simply pass a mobile reader over it to read your glucose levels. It even works for 10 days before you have to replace it.

Apple, Alphabet and Fitbit test FDA fast track for health apps
A few of the tech giants you know are about to get fast-track approval for their digital health efforts. The US Food and Drug Administration has named the companies involved in a recently-instituted "pre-certification" program that determines whether or not they meet baseline quality standards for health software. Apple, Fitbit, Samsung and Alphabet's Verily are among the firms that will help the FDA set the benchmarks and decide just how much information companies need to send if they've been pre-cleared. Depending on how the program fares, companies may get to send less information or even avoid certifying certain apps altogether.

Natural Cycles says contraceptive app is more effective than the pill
Contraceptive app Natural Cycles is more effective than the pill, according to the latest and largest study into the app's efficacy. After testing 22,785 women throughout 224,563 menstrual cycles, the startup found the app provided 99 percent contraceptive effectiveness if used perfectly. If used "typically", the app was 93 percent effective. The contraceptive pill, meanwhile, is 91 percent effective.

FDA recalls close to half-a-million pacemakers over hacking fears
Turns out former Vice President (and erratic shooter) Dick Cheney was right all along: Your heart can be hacked. At least if you have a pacemaker, that is. On Tuesday, the FDA recalled 465,000 of the medical devices -- the ones that help control your heart beat -- citing security vulnerabilities. The pacemakers, which come from health company Abbott (formerly St. Jude Medical), require a firmware update. Fortunately, it can be installed by a health care provider in just three minutes. The models affected include the Accent, Anthem, Accent MRI, Accent ST, Assurity, and Allure.

First FDA-approved genetic therapy fights leukemia
The first gene therapy treatment has been approved for use in the United States. The FDA greenlit a procedure that uses a patient's own cells to combat a particular type of leukemia, but will only permit it for children and young adults up to age 25.

FDA hasn't confirmed if meatless Impossible Burger is okay to eat
The meatless Impossible Burger has been hard to track down since it debuted last August. Its parent company Impossible Foods promised that a massive new factory would put the faux beef patties in 1,000 restaurants by the end of the year, but that rollout might get hampered by the FDA. The agency isn't sure about what's in the meat substitute: It turns out the ingredient that makes the Impossible Burger look, taste and (er) bleed like beef hasn't been consumed by humans before, and could be an allergen that potentially provokes allergic reactions.

The FDA has a significant change of heart about e-cigarettes
The FDA has just announced a sweeping change in its policy regarding e-cigarettes and vaping products. In a press release issued this morning, the administration outlined its plan to focus on reducing usage of combustible cigarettes and tobacco, in turn loosening restrictive rules laid out just last year, that could have wiped out most vaping products ("eliquid").

FDA approves first MRI machine for premature babies
Premature babies are some of the most vulnerable patients in a hospital, and they also need some of the most dedicated care. Treating these tiny patients in the neonatal unit, or NICU, can be a challenge, especially when it comes to magnetic resonance imaging (MRIs). Taking a baby through the hospital to an MRI machine and exposing them to germs is not a decision a doctor takes lightly, and that's why this new announcement is a welcome one: The FDA has cleared a new MRI machine for exclusive use in neonatal units.

High-tech solutions top the list in the fight against eye disease
"The eyes are the window to the soul," the adage goes, but these days our eyes could be better compared to our ethernet connection to the world. According to a 2006 study conducted by the University of Pennsylvania, the human retina is capable of transmitting 10 million bits of information per second. But for as potent as our visual capabilities are, there's a whole lot that can go wrong with the human eye. Cataracts, glaucoma and age-related macular degeneration (AMD) are three of the leading causes of blindness the world over. Though we may not have robotic ocular prosthetics just yet, a number of recent ophthalmological advancements will help keep the blinds over those windows from being lowered.

New FDA policies could hasten approvals of 'lower risk' health tech
The FDA is making an effort to keep up with the digital world. The commissioner of the FDA said in a statement yesterday that the agency would be outlining ways to streamline the regulation of digital health devices.

FDA approves a more 'personalized' cancer drug
Cancer treatments are becoming more personal. The Food and Drug Administration recently gave accelerated approval for Keytruda, a pre-existing drug from Merck, for use on patients diagnosed with solid tumors containing a specific biomarker. Rather than basing treatment on where the mutation originated, Keytruda will be used to treat microsatellite instability-high (MSI-H) cancers, those that are mismatch repair deficient (dMMR) and are otherwise not able to be surgically removed. These types of tumors affect how the DNA is repaired inside the cell.

FDA: Stop buying miracle cancer drugs on Instagram
The FDA has issued a warning to social media users not to be taken in by miracle cures hawked on the internet. The agency found 14 companies that made fraudulent, outrageous claims about the power of their medicines on platforms like Facebook and Instagram. Marketed as "treatments," and often described as "natural," these pills are likely to do more harm than good to people undergoing treatment.
FDA clears 23andMe to warn you about potential health risks
Good news for everyone who wants to do at-home DNA tests: 23andMe has been cleared by the Food and Drug Administration to tell customers if they're at risk for 10 potentially debilitating diseases. "These are the first direct-to-consumer tests authorized by the FDA that provide information on an individual's genetic predisposition to certain medical diseases or conditions, which may help to make decisions about lifestyle choices or to inform discussions with a health care professional," the watchdog agency said in a statement. Previously, the FDA had stopped the company from offering this information back in 2013. The FDA softened its stance a bit in 2015.
Old drug gets an absurd price hike to $89,000 per year
Martin Shkreli doesn't have a monopoly on jacking up drug prices. Marathon Pharmaceuticals has received FDA approval for an old drug used to treat Duchenne muscular dystrophy, deflazacort, and in the process has raised the price to ridiculously high levels. You could previously import the generic medicine for about $1,200 per year, but it now costs a staggering $89,000 per year -- "just" $54,000 with discounts and rebates. And since deflazacort (now rebranded as Emflaza) is classified as an orphan drug used to treat a rare disease, Marathon will both have a 7-year exclusive on US sales and a voucher to fast-track a future approval.

FDA warns that certain pacemakers are vulnerable to hacking
According to a cybersecurity notice from the Food and Drug Administration, certain pacemakers and cardiac devices are currently vulnerable to hacking. Although security researchers have warned about the security risks to medical devices for years now, this is the first time we've seen the government publicly acknowledge a specific threat.

FDA issues final guidance on medical devices' cybersecurity
The Food and Drug Administration has issued its final guidance on protecting medical devices like pacemakers and insulin pumps from cyberattacks. To start with, it wants manufacturers to boost their cybersecurity measures by incorporating a way to monitor and detect vulnerabilities into the products they make. The FDA also wants them to establish a process for receiving information about potential vulnerabilities from cybersecurity researchers. If they do detect any exploitable flaw, the agency wants the companies to assess the risk it poses to patients. Finally, it wants the medical device makers to issue software patches to fix any vulnerability it finds.

Philip Morris submits a tobacco vaporizer for FDA approval
Philip Morris, maker of Marlboro cigarettes, submitted an application to the FDA on Tuesday seeking approval for its new tobacco vaporizer. The iQOS device, as it's currently called, works on the same principle as the Pax, wherein the ground plant matter is gently heated until the active ingredients are vaporized, rather than burned with an open flame. Philip Morris claims that the vapor has 90 percent fewer harmful chemicals than normal cigarette smoke.










