fda
Latest

Parents may be able to spot ear infections with a paper cone and an app
Researchers are working on a smartphone app that could help diagnose ear infections. As NPR reports, the app uses the phone's microphone, its speaker and a small paper cone. In its current form, the app sends short, sound pulses through a funnel and into the ear canal. It then measures the echo of that sound, and an algorithm uses the reading to predict if there's fluid behind the eardrum, one of the common symptoms of infection.
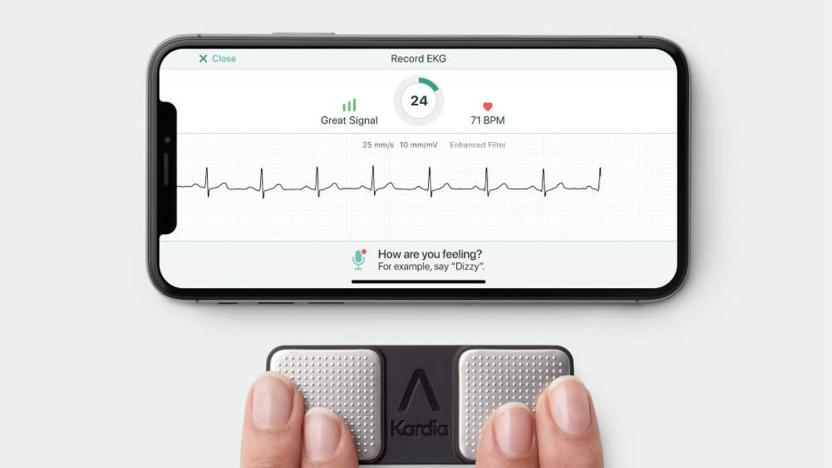
FDA clears first personal ECG device to detect three heart arrhythmias
To date, personal ECG devices have only really detected one kind of heart arrhythmia: atrial fibrillation. While that's helpful, it doesn't cover other conditions that could be just as dangerous. You might not be left wondering for much longer. AliveCor's KardiaMobile has received the first FDA clearance allowing a personal device to detect two other relatively common conditions, bradycardia (where your heart rate dips to 40-50BPM) and tachycardia (a jump to 100-140BPM). While these conditions are sometimes innocuous and might not show symptoms, they can also be representative of issues like heart disease.

The FDA thinks an Xbox game can stop kids smoking
The FDA is behind a horror video game designed to teach teens about the dangers of smoking. Inspired by the statistic that three out of every four high school students who start smoking continue on to adulthood, One Leaves sets players in a cell with three other teens. The free PC and Xbox One game's objective is to escape the cell, but only one of the players will succeed. The game is being released as part of the FDA's "The Real Cost" youth tobacco prevention campaign, which is aimed at youth who are 12 to 17 years of age.

San Francisco lawmakers will consider a ban on e-cigarette sales
Authorities in San Francisco are considering banning the sale of e-cigarettes until the FDA carries out an investigation on their effects on health. Officials from the city -- which has already banned the sale of flavored tobacco and flavored vaping liquid -- said such a review should have been completed before e-cigarettes entered the market.

FDA proposes stricter rules for flavored e-cigarettes
After months of talking about limiting flavored e-cigarettes, the FDA ready to take more definitive action. The regulator has unveiled draft rules that would let it restrict sales of e-cigs with flavors that could appeal to kids (that is, everything outside of menthol, mint and tobacco). It would "prioritize" enforcement on sales of those flavors in ways that make them easily accessible to or enticing for kids. This could include retail stores where kids can walk in at any time, online stores with weak quantity limits and products whose look and flavor could appeal to the younger crowd.

FDA removes restrictions on genetically modified salmon
Genetically modified fish are about to become more of a practical reality in the US. The Food and Drug Administration has lifted an import alert on AquaBounty's genetically modified AquAdvantage salmon eggs, allowing the fish to reach the US over three years after they received initial approval. Congress told the FDA in 2016 to block modified salmon until it issued labeling guidelines, and the Administration believes Congress' newly-enacted National Bioengineered Food Disclosure Standard meets that criteria.
FDA: Infusing yourself with young blood is pointless, dangerous
The US Food and Drug Administration has stepped in to officially warn consumers against buying young blood in an attempt to improve their health. Yes, enough people thought it would be a good idea to infuse young blood -- an actual plot from The Simpsons' -- that the government had to intervene.

FDA explores using blockchain to track drug supplies
The US Food and Drug Administration wants to be sure sketchy drugs don't find their way to hospitals and pharmacies, and it's mulling a technological solution to keep medicine safe. The agency has launched a pilot program that will let the drug supply chain explore ways to track prescription medicine. While the FDA isn't specific about what tech companies would use, it noted that blockchain was one example. The same decentralized trust system that can trace the origins of your lettuce could also verify that your pills come from a legitimate source.

FDA accuses Juul of undermining efforts to prevent teen vaping
Many people raised eyebrows when Marlboro owner Altria bought a $12.8 billion stake in the vaping giant Juul, and that now includes the US Food and Drug Administration. Commissioner Scott Gottlieb has requested a joint meeting with the CEOs of Juul and Altria over concerns their statements "contradict" commitments they made in October to reduce teen vaping. The official wanted both executives to explain how their deal affects their plans to curb youth vape use, and noted that data suggested trends were headed in the wrong direction.

Shutdown means government won't engage with the tech industry at CES
It's not just FCC Chairman Ajit Pai who'll back out of CES as a result of the US government shutdown. The Consumer Technology Association has confirmed that at least ten government officials have cancelled their speaking engagements at the technology trade show "so far." It's not just FCC representatives like Pai or his compatriot Brendan Carr, either. The FTC's Rohit Chopra and Rebecca Slaughter (shown above) have backed out, as have officials from the EPA (Brandon Bray and Barnes Johnson), FDA (Bakul Patel), FEMA (Daniel Kaniewski) and Homeland Security (Andre Hentz).

FDA approves app-connected digital inhaler
The FDA has given the ProAir Digihaler, a digital and mobile-connected inhaler that comes with built-in sensors, its sweet approval. Those sensors can detect whenever the device is used and even measure the strength of the user's inhalation. More importantly, the inhaler can send data on people's usage to its mobile app companion. By providing them with visual information, users can spot trends and ensure they're getting the right amount of medication, as well as share it with their doctors if needed.

FDA to limit sales of flavored e-cigarettes
The Food and Drug Administration is essentially banning flavored e-cigarette sales at convenience stores and gas stations as part of a crackdown on underage vaping and smoking. The agency is limiting sales in physical stores to those that have age-restricted entry or areas that are closed off to under-18s. It will also require more vigorous age verification for online sales. It was widely expected that the FDA would take action against flavored e-cigarettes, though it stopped short of a complete ban.

Juul stops selling flavored e-cigarette pods, kills social media accounts
Juul Labs, the makers of the wildly popular Juul e-cigarettes, announced today that it will stop selling most of its flavored vaping pods in retail stores. The company will also put an end to its social media promotions and advertisements. The decision on the part of Juul comes as the government appears ready to apply more scrutiny to the vape brand and its potential targeting of kids.

FDA to ban flavored e-cigarette sales in convenience stores
You might have a hard time finding flavored e-cigs outside of vape shops in the future. According to The Washington Post, the Food and Drug Administration plans to announce a ban on the sales of most pod-based flavored e-cigarettes in convenience stores and gas stations across the country. It's reportedly part of the agency's efforts to curb sales of e-cigs to minors, since studies show that flavors tend to attract underage buyers. The FDA says that there's been a 77 percent increase in the use of e-cigarettes among high school students in 2018 -- Juul has been particularly popular among teens to the point that vaping with Juul is now commonly known as "juuling."
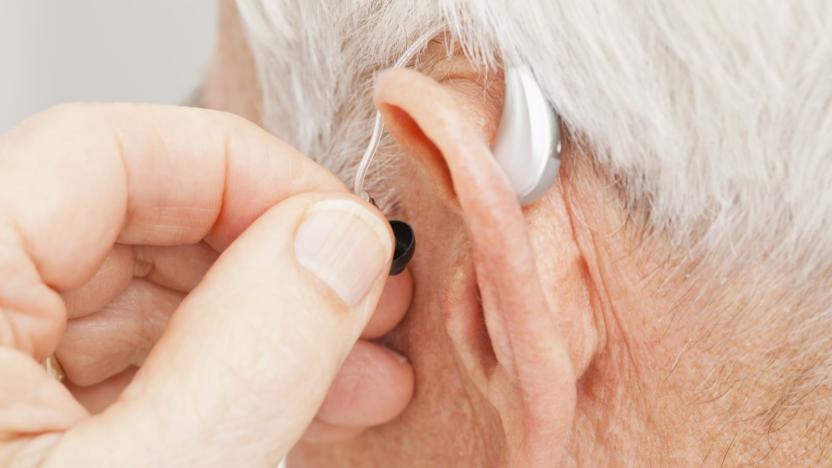
FDA approves direct-to-consumer hearing aid from Bose
The US Food and Drug Administration has, for the first time, approved a hearing aid that can be fit, programmed and controlled by the user instead of a healthcare provider. The device comes from Bose and users can make adjustments to its settings in real time through a mobile app. "Hearing loss is a significant public health issue, especially as individuals age," said Malvina Eydelman, director of the Division of Ophthalmic, and Ear, Nose and Throat Devices at the FDA's Center for Devices and Radiological Health. "Today's marketing authorization provides certain patients with access to a new hearing aid that provides them with direct control over the fit and functionality of the device. The FDA is committed to ensuring that individuals with hearing loss have options for taking an active role in their health care."

FDA seizes marketing documents from e-cig maker Juul
In its latest move against e-cigarettes, the US Food and Drug Administration has seized "thousands of pages of documents" from e-cig maker Juul. In a statement to CNBC, the FDA said the action was taken in order to obtain "further documentation related to Juul's sales and marketing practices, among other things." The documents were seized last week during a surprise inspection of the company's San Francisco headquarters.

E-cig makers have 60 days to show they aren’t targeting minors
The Food And Drug Administration may force several e-cigarette brands to stop selling flavored products if they can't prove they can keep their products out of minors' hands. The brands -- Juul, Vuse, MarkTen, blu and Logic -- have 60 days to convince the agency they have adequate plans to stop kids from vaping with their products. Those five collectively account for more than 97 percent of the e-cigarette market.

FDA and USDA will meet to debate the future of lab-grown meat
It's inevitable that lab-grown meat will play some kind of role in the future of food supply, but at this stage, it's unclear how much of a role, or what its regulatory frameworks will look like. This is why the US Food and Drug Administration (FDA) and US Department of Agriculture (USDA) are hosting a joint public meeting on the issue, to address public concerns about cell-cultured meat products and to examine how they will fit into existing food systems.

FDA approves contraceptive app Natural Cycles
The FDA has now granted marketing approval to an app that tracks a user's temperature and menstrual cycle in order to determine which days they are fertile and which days they aren't. The contraceptive app, called Natural Cycles, was approved in Europe last year. "Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it's used carefully and correctly," said Terri Cornelison, assistant director for the health of women at the FDA's Center for Devices and Radiological Health. "But women should know that no form of contraception works perfectly, so an unplanned pregnancy could still result from correct usage of this device."

FDA says unapproved 'designer vagina' treatments are dangerous
Cellulite, thigh gaps, hip dips... women have no end of supposed physical "flaws" to worry about, and in recent times this remit has expanded to include the state of their vaginas, too. The internet is awash with products designed to improve a woman's "intimate health", but now the FDA has found that these treatments and procedures -- which claim to tighten muscles, increase lubrication, boost sexual pleasure or just "neaten things up" -- are not only unapproved, but are causing serious injuries to the women undertaking them.







