fda
Latest

FDA approves new trials using MDMA to help treat PTSD
MDMA, the pure form of Ecstasy, is usually only mentioned when partygoers get busted for holding the Schedule 1 restricted drug. But medical researchers have been quietly testing its potential medical applications, like treating terminally ill patients and assisting patient therapy for PTSD. The results from the latter have been promising enough for the FDA to commission Phase 3 trials, which is the last step before MDMA's possible approval as a prescription drug.

Florida voters conflicted over Zika-fighting mosquitoes
You can't just go and release genetically-modified mosquitoes into the environment without making sure people are okay with it -- regardless of how badly we need a way to eradicate the Zika virus. In Tuesday's election, 65 percent of Florida residents in the city of Key Haven voted against a ballot measure that'd sanction such a test, according to MIT Technology Review. Meanwhile, some 58 percent of voters in the county that Key Haven is a part of, Monroe County, voted in favor of the test. Now it's up to the Florida Keys Mosquito Control District Board of Commissioners (FKMCDBC) to make a decision.

FDA approves first automated insulin system for type 1 diabetes
For diabetes patients, managing blood sugar levels through insulin pens, needles or pumps is a necessary hassle -- but it might be far easier to handle going forward. The US Food and Drug Administration has approved the first automated insulated delivery device for type 1 diabetes, Medtronic's MiniMed 670G. The gadget uses a sensor to detect glucose levels under your skin every 5 minutes, and supplies just enough insulin to keep your blood sugar stable. While you do have to trigger a manual insulin dose after meals, you generally won't have to be as involved in the process as before.

Theranos' Zika test is under FDA scrutiny
That didn't take long. Theranos pulled the emergency request for its Zika-testing miniLab following an FDA inspection that revealed shortcomings with patient protocol, according to The Wall Street Journal. WSJ's sources say that while the blood-testing company had data showing that the testing methods worked, the evidence collected was done without "implementing a patient-safety protocol approved by an institutional review board." Meaning, the experiments didn't have the type of oversight necessary to ensure that they were performed in stringent, controlled ways that would consistently provide accurate results and wouldn't harm the test subjects. The company says that it "recognized" that some of its data was gleaned before the needed protocols were in place. Theranos plans to appeal the Food and Drug Administration's ruling, of course, and collect the extra information requested by the FDA before trying the certification process again. Same goes for when the company attempts certification for its Ebola test as well. The miniLab itself was a way to sidestep founder Elizabeth Holmes' two-year ban from owning or running a lab or her own, precisely because the device was made for outside use. All of this raises the question of how long Jennifer Lawrence will have to wait for a final script for Theranos: The Movie. Seriously, this saga just keeps getting better.

FDA recommends that all donated blood be tested for Zika
In light of the Zika virus rapidly spreading to other parts of the world, the Food and Drug Administration has changed up its recommendations for donated blood. Going forward, all blood donated in the United States should be screened for the Zika virus.

Starting today, it will be a lot harder to vape if you're under 18
In May, the Food and Drug Administration (FDA) announced plans to regulate e-cigarettes like it does regular tobacco products. Today, those changes go into effect. First, the new regulations make it illegal to sell e-cigarettes and other vaping supplies to anyone under the age of 18. As we reported when the FDA first revealed its plans, the age limit was already being enforced in some places, but now it's the rule nationwide. Retailers will be required to ask for identification from any customer who appears to be under the age of 27 and are prohibited from providing free samples to minors.
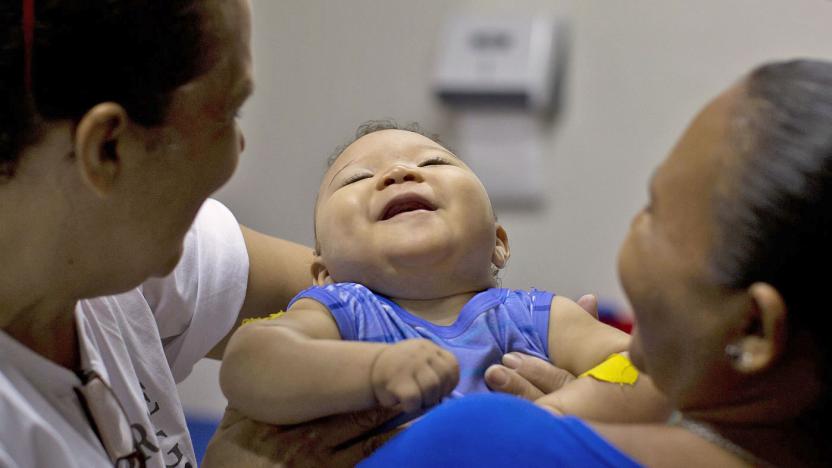
Experimental Zika vaccine approved for human trials
The Food and Drug Administration approved an experimental Zika vaccine called GLS-5700 for a clinical trial in humans earlier today, the first such treatment to get an official nod from the agency. Initial trials will start with 40 healthy subjects getting dosed in the coming weeks, and if all goes without a hitch, preliminary results should be available later this year.

ICYMI: Rock-like smartphone, stomach tap and more
try{document.getElementById("aol-cms-player-1").style.display="none";}catch(e){}Today on In Case You Missed It: The FDA has just approved a device for obese people that is first surgically inserted into the stomach, then used like a tap after meals to drain up to a third of the food inside. The Runcible 'anti-smartphone' is going up for sale for $300, designed to not make a single noise except to notify you of incoming calls. It includes a camera, bluetooth and touchscreen, but still clearly resembles a rock on the back. And finally, It is this show's first birthday, so we are touching on a few of our favorite stories from the last year. If you'd like to check out a brief clip of the pigeon video out of New York, that's here. As always, please share any interesting tech or science videos you find by using the #ICYMI hashtag on Twitter for @mskerryd.
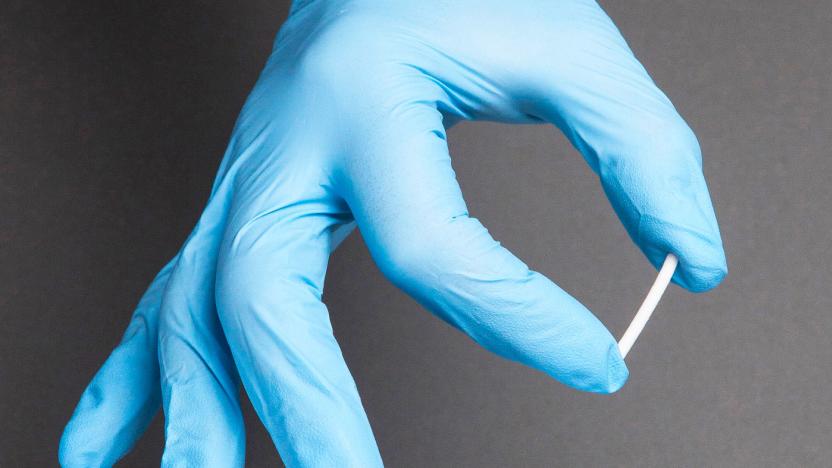
FDA OKs first implant treatment for opioid addiction (updated)
That small stick in the image above might look like a fat toothpick or a part of a toy that broke off, but its much, much more than that. It's called Probuphine, and it's the first implant treatment for opioid addiction that got the FDA's blessing. Opioid dependence is a huge problem today, especially since opioids encompass not just illegal substances like heroin, but also legal pain killers, such as those prescribed after surgery.

The After Math: Regulation Nation
Exciting news coming from the wild world of government rule making this week. The FDA signed off on a medical experiment designed to jumpstart your brain after it's died. Surprisingly, no, lead-acid batteries are not involved. The FDA also made waves by denying minors the ability to buy e-cigarettes, prompting calls from the public for the agency to explain why it took so damn long to do so. The FAA relaxed its rules over drone swarms, Takata pissed off the DoT yet again and Tesla made the EPA look a little silly. Numbers, because the Feds said so.

FDA will regulate e-cigarettes like tobacco products
As the debate over the health risks of e-cigarettes rages on, the FDA is stepping in to "improve public health and protect future generations." To do that, the US government will regulate e-cigs and vaping gear like it does any other tobacco product. Until now, these products haven't been subject to government oversight. With the FDA's changes, the federal law that already forbids tobacco sales to people under 18 will now apply to vaping as well. Sure, this age limit was already being enforced in some places, but this more formal announcement makes it a nation-wide law.

FDA approves 'world's smallest' pacemaker for heart patients
As consumer technology has trended smaller and thinner, medical devices have done the same. And now, the first transcatheter pacemaker has been approved for use with heart patients in the US. Medtronic gained FDA approval for its Micra TPS pacemaker, the first device to employ the miniaturized pacing tech to be approved by the US government. The company is calling the device the "world's smallest pacemaker," measuring just a tenth of the size of traditional technology. It's about the size of large vitamin.

ICYMI: Fast brain upload, mind-control monkeys & more
#fivemin-widget-blogsmith-image-222051{display:none;} .cke_show_borders #fivemin-widget-blogsmith-image-222051, #postcontentcontainer #fivemin-widget-blogsmith-image-222051{width:570px;display:block;} try{document.getElementById("fivemin-widget-blogsmith-image-222051").style.display="none";}catch(e){}Today on In Case You Missed It: Researchers at HRL Labs have developed a system to upload information to your brain using electrical signals already mapped from an expert's mind. Duke University is testing a wireless brain-machine interface that allows monkeys to steer a wheelchair with their mind, which they were able to do while also improving their skills over time. Cardiologists have a new tool to roto-rooter blood vessels filled with plague in the first FDA approved device that helps surgeons see inside vessels with a built-in camera.

Walgreens has told Theranos to shape up or ship out
The Wall Street Journal is reporting that the relationship between Walgreens and Theranos is becoming fractious. According to the paper's unnamed sources, the drugstore has threatened to end its partnership with the troubled blood-testing startup. It's believed that Walgreens delivered an ultimatum to the firm in late January, saying that it needs to clean up its act within 30 days or be kicked to the curb. If true, then we could see the two part ways as early as the end of February. Walgreens is already doing its best to distance itself from its former BFF after shutting down the Theranos Wellness Center in Palo Alto and re-routing Theranos-branded tests to third-party labs.

The FDA wants improved cybersecurity for medical devices
The Food and Drug Administration has released draft cybersecurity guidelines for medical device makers. It still remains only a guideline, but data leaks and security issues are typically never a good thing for a company -- especially when lives are literally on the line. The draft suggests that companies monitor and assess cybersecurity risks (like hacking or data leaks), as well as coordinate information sharing between companies and government to help fix or address vulnerabilities as quickly as possible.

Former employee claims Theranos' FDA-approved test is faulty
Startup medical testing company Theranos came under fire in October as the accuracy of its tests were called into question. The FDA has been investigating since then, and today more fuel was thrown on the fire by some former employees. According to The Wall Street Journal, both the FDA and the Centers for Medicare and Medicaid Services (CMS) each received a complaint from a different former employee. The FDA received notice that the study Theranos submitted to win the agency's approval for its herpes test was "tainted by breaches in research protocol." That's notable because so far that herpes test is the only one of Theranos' tests that has actually been approved by the FDA. Founder Elizabeth Holmes (above) has continued to deny any accusations claiming that Theranos' data is inaccurate.

A sponge-filled syringe could save you from bleeding out
RevMedx's sponge-filled syringe, the XSTAT 30, was approved for military use in treating gunshot wounds last year. Now, the FDA says paramedics and other first responders can use the device to treat civilian injuries as well. The syringe is filled with tiny sponges that are designed to control severe bleeding from wounds in places a tourniquet can't be used. Each syringe contains 92 compressed sponges that expand to fill the wound to block blood flow for up to four hours.

World's first in-human gene-editing treatment will tackle hemophilia
Hemophilia B is a terrifying disease. The livers of those suffering from the genetic disorder fail to produce a key protein called Factor IX, which is responsible for clotting blood. Without this protein, they're at constant risk of uncontrollable bleeding, including internally. However, a pair of researchers believe that their novel gene therapy could permanently cure the disease. To that end, the team of Michael Holmes and Thomas Wechsler from Richmond, California's Sangamo biopharmaceuticals, have announced that the world's first in-patient gene-editing therapy targeting these faulty genes will commence next week.

FDA approves world's first GMO fish: fast-growing Atlantic Salmon
In a landmark decision more than two decades in the making, the US Food and Drug Administration announced its approval of a genetically modified Atlantic Salmon variant on Thursday. The AquAdvantage salmon, which was initially developed back in 1989 and submitted for approval in 1995, grows far faster than its conventionally bred brethren. The FDA has deemed it safe for human consumption, equally nutritious as other salmon varieties and not dangerous to the environment. And since the GMO salmon is considered nutritionally equivalent to regular salmon supermarkets will be able to carry the fish without having to label them being GMO.

23andMe given permission to offer some limited health reports
23andMe has been given the green light to resume some, but not all, of the health reporting that the FDA prohibited it from carrying out in 2013. The startup launched with the pitch that a sample of your saliva was enough to tell you where your ancestors came from as well as if you would go bald in old age. Controversially, the company also told you if you were at risk of a wide variety of diseases, but never asked for permission to do so. That's why regulators shut it down, since the outfit was unable to supply the data to prove that the tests were accurate.











